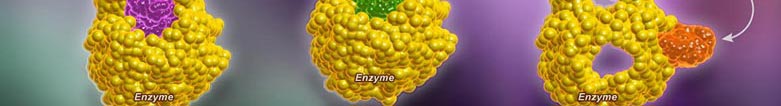
Enzyme inhibitors prevent the formation of an enzyme-substrate complex and hence prevent the formation of product. Inhibition of enzymes may be either reversible or irreversible depending on the specific effect of the inhibitor being used.
In a normal reaction, a substrate binds to an enzyme (via the active site) to form an enzyme-substrate complex. The shape and properties of the substrate and active site are complementary, resulting in enzyme-substrate specificity. When binding occurs, the active site undergoes a conformational change to optimally interact with the substrate. This conformational change destabilizes chemical bonds within the substrate, lowering the activation energy. As a consequence ofthis enzyme interaction, the substrate is converted into product at an accelerated rate.
A competitive inhibition involves a molecule, other than the substrate, binding to the enzyme’s active site. The molecule (inhibitor) is structurally and chemically similar to the substrate (hence able to bind to the active site). The competitive inhibitor blocks the active site and thus prevents substrate binding. As the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration.
A non-competitive inhibition involves a molecule binding to a site other than the active site (an allosteric site). The binding of the inhibitor to the allosteric site causes a conformational change to the enzyme’s active site. As a result of this change, the active site and substrate no longer share specificity, meaning the substrate cannot bind. As the inhibitor is not in direct competition with the substrate, increasing substrate levels cannot mitigate the inhibitor’s effect.
Drugs that function as enzyme inhibitors constitute a significant portion of the orally bioavailable therapeutic agents that are in clinical use today. Most of the drug discovery and development efforts at present are focused on identifying and optimizing drug candidates that act through inhibition of specific enzyme targets. The attractiveness of enzymes as targets for drug discovery stems from the high levels of disease association (target validation) and druggability (target tractability) that typically characterize this class of proteins.
Aurora Medbiochem is proud to offer a variety of structurally diverse enzyme inhibitors for research.
Potent inhibitor of PARP-1 and PARP-2 (potency ≤5 nM in vitro).
A specific, irreversible serine protease inhibitor. Inhibits proteases like chymotrypsin, kallikrein, plasmin, thrombin and trypsin. A stable, non-toxic and better soluble alternative to PMSF.
AG-1295 is a selective platelet-derived growth factor receptor (PDGFR) tyrosine-kinase inhibitor.
A potent thiadiazolyl analog that binds to PH (pleckstrin homology) domain (Kd = 40.8 µM, Ki = 2.4 µM)..
A cell-permeable compound that directly and reversibly interacts with miRNA binding domain of Argonaute-2 (Kd = 126 µM) and inhibits binding of miR-20a, miR-26a, miR-107, miR-223 & let-7a to Ago2.
AS 1842856 is a potent and selective Foxo1 inhibitor (IC50 values are 33 nM at Foxo1 and >1 μM at Foxo3a and Foxo4)
Atglistatin is a potent, selective, and competitive inhibitor of ATGL (IC50 = 0.7 μM). It does not inhibit hormone-sensitive lipase, monoglyceride lipase, pancreatic lipase, lipoprotein lipase, or other lysophospholipases.
Bestatin is a protease Inhibitor that reversibly inhibits amino peptidases.
BLT-1 is a novel inhibitor of scavenger receptor BI (SR-BI).
A01 is a high affinity Smurf1 E3 ubiquitin ligase inhibitor (Kd = 3.7 nM). Attenuates Smurf1-mediated Smad 1/5 degradation and enhances BMP signaling. Potentiates BMP-2-induced osteoblastic activity in C2C12 myoblasts and MC3T3-E1 osteoblast precursor cells.
Bortezomib is a cell-permeable dipeptidylboronate compound that acts as a potent and selective 26S proteasome inhibitor. Human pancreatic cancer cell studies demonstrate Bortezomib to inhibit the PKR-like endoplasmic reticulum (ER) kinase and enhance ER stress, leading to apoptosis.
Bosutinib (SKI-606) is a novel tyrosine kinase inhibitor. Bosutinib can overcome not only Bcr-Abl-dependent mechanisms of resistance, but also those that are Bcr-Abl-independent. It is used to treat patients with chronic myelogenous leukemia (CML) who demonstrate resistance to Imatinib or develop resistance during treatment.